

This information is intended for US healthcare professionals.

First–in–class capsid inhibitor that targets HIV–1 at multiple stages of the HIV–1 life cycle1,3
The capsid core contains and protects viral RNA and enzymes, all of which play essential roles in HIV-1 replication. The HIV-1 viral life cycle is dependent on the function of the capsid at the following stages of HIV-1 replication: nuclear transport, virus assembly and release, and capsid core formation.1,3-8
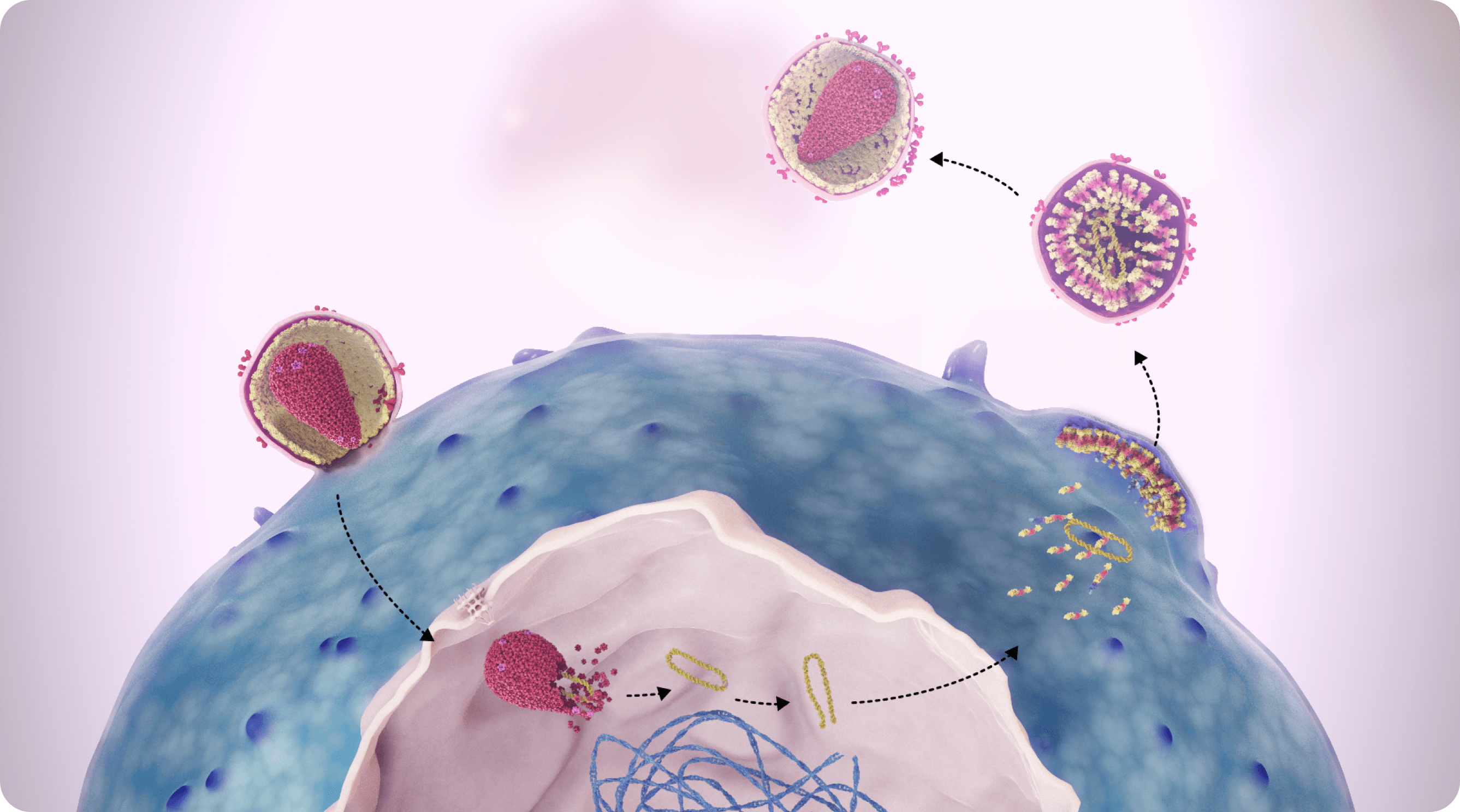
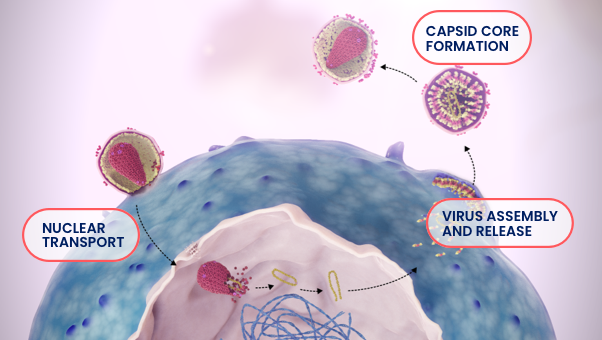
SUNLENCA Interferes With 3 Essential Steps of HIV-1 Viral Replication1,3,4
Important Safety Information
Contraindications
SUNLENCA provided full activity in vitro against mutants resistant to other ARVs, including INSTIs, NNRTIs, NRTIs, and PIs.1,3
SUNLENCA Is Fully Active Against Variants With the Following Gene Mutations That Cause ARV Resistance1,3,9


SUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 whose current antiretroviral regimen is failing due to resistance, intolerance, or safety considerations.
Contraindications
Warnings and precautions
SUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 whose current antiretroviral regimen is failing due to resistance, intolerance, or safety considerations.
Tap for Important Safety Information
Contraindications
Warnings and precautions
SUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with multidrug resistant HIV-1 whose current antiretroviral regimen is failing due to resistance, intolerance, or safety considerations.